Want to stop being the invisible manufacturer behind European brands? You’re making world-class surgical instruments in Sialkot, but someone else is slapping their label on your work and keeping the big profits.
Here’s the truth—you can export medical devices to Qatar directly and capture those margins yourself.
We’ve helped 19+ businesses launch ventures across the Pakistan-GCC corridor. While timelines vary, one exceptional client was able to land a $200,000 deal within just 90 days of finalizing their registration. Qatar’s healthcare market is growing fast, with a national healthcare budget exceeding $7 billion annually. This massive investment fuels a continuous demand for imported medical supplies across hospitals and clinics. Whether you make surgical scissors, forceps, scalpels, or dental instruments—there’s continuous demand across hospitals, clinics, and surgery centers.
This guide uses disposable surgical scissors as the teaching example, but every step applies to your entire product range. Let’s break down exactly how you can build a real export business—no hype, just the practical steps you need.
Why Qatar Is Your Best First Move
You might be thinking, “Why not Dubai or Saudi Arabia? They’re bigger markets.” True. But here’s what makes Qatar your smart starting point.
Qatar has one major buyer controlling 80% of public hospitals—Hamad Medical Corporation. Win one relationship, and you’ve got access to the entire healthcare network. Compare that to Dubai or Riyadh, where you’d be chasing dozens of different hospital groups.
Plus, Qatar has among the highest healthcare spending per capita in the GCC region, with government healthcare expenditure reaching over $2,300 per person. That’s serious buying power for quality medical supplies.
The registration process is also more straightforward. Qatar’s Ministry of Public Health moves faster than Saudi’s SFDA, especially for basic surgical instruments like disposable scissors. You’ll get approved in two to four months instead of waiting six months or longer.
Why We’re Using Surgical Scissors as Your Example
Here’s something important—this entire roadmap works for ANY medical device you manufacture. Surgical forceps, scalpel handles, retractors, needle holders, dental instruments—they all follow the same compliance path.
We’re focusing on disposable surgical scissors because they’re the perfect teaching example. They’re simple, high-demand, and easy to understand. Once you’ve cracked the code with scissors, you can apply the same framework to your entire product catalog.
Every hospital, clinic, dental office, and surgery center needs surgical scissors. They use hundreds every week because you can’t reuse them—that’s a hygiene rule. This means steady, predictable demand that doesn’t stop.
They’re classified as Class I medical devices, which is the simplest category to register. You won’t need complex clinical trials or years of testing. Get your paperwork right, and you’re approved.
Sialkot manufacturers already make 70-80% of the world’s manual surgical instruments, employing over 100,000 skilled workers. You’ve got the skills, the materials, and the production lines. You just need the compliance framework to sell directly to hospitals instead of through middlemen.
The beauty? Once you’ve built your ISO 13485 certification, DRAP licenses, and Qatar distributor relationships for scissors, adding more products costs a fraction of the initial investment. Your compliance infrastructure becomes a reusable asset across your entire portfolio.
The Real Costs You Need to Know
Let’s talk money. No point hiding the numbers.
Your total startup investment will run between $30,000 and $45,000. Here’s where it goes:
Quality certifications: You’ll spend $6,000 on ISO 13485 certification. This proves your factory meets international medical device standards. Every serious buyer checks for this first. Add another $3,000 for developing your CE technical file—the document package proving your scissors are safe and effective.
Pakistan compliance: DRAP licensing costs about $2,000. This is your legal authorization to manufacture and export medical devices from Pakistan. No shortcuts here—customs will block shipments without it.
Sterilization setup: Budget $4,000 for sterilization validation. Your scissors must arrive sterile at hospitals. You’ll work with facilities in Karachi or Lahore using ethylene oxide gas or gamma radiation. Conducting thorough feasibility studies before committing helps you understand these cost structures clearly.
Qatar registration: Another $3,000 goes to registering your product with Qatar’s Ministry of Public Health through your local distributor.
First production run: You’ll need $8,000 for initial manufacturing inventory—enough to fill your first few orders and have samples for hospital trials.
Market entry support: Reserve $4,000 for travel, exhibition booths, and relationship building. You can’t sell from behind a computer screen.
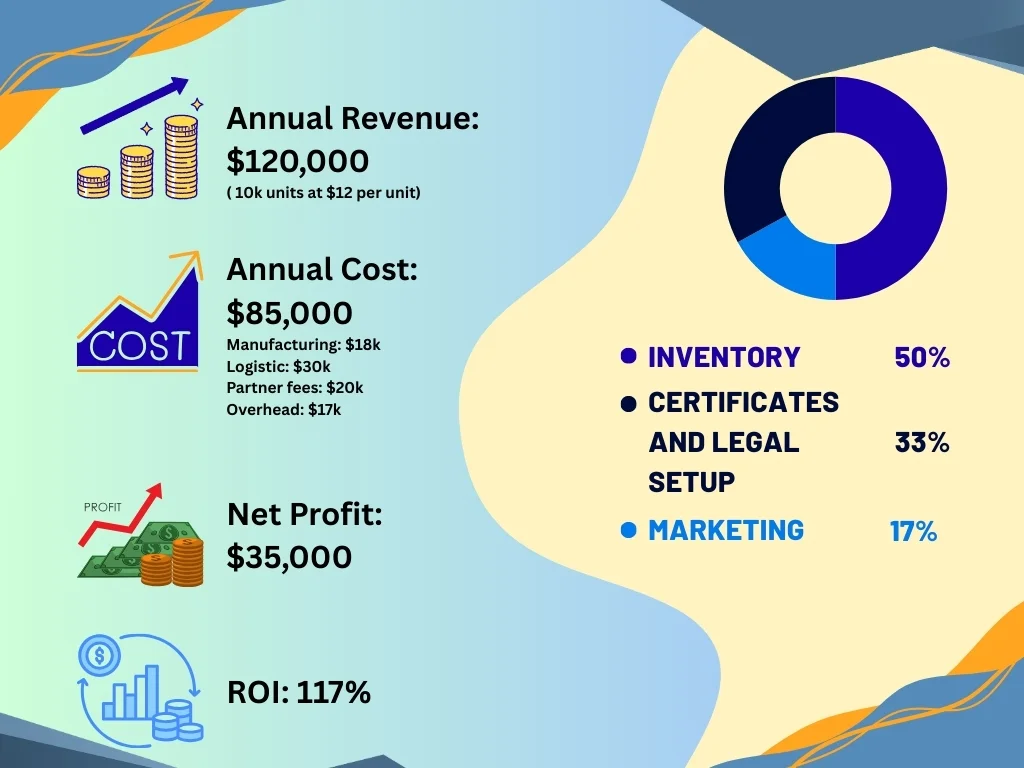
Now, here’s your return. Each scissor costs you $1.15 to $1.80 to make (including sterilization and packaging). You’ll sell them to Qatar distributors for $4.00 to $7.00. European suppliers charge hospitals $8.00 to $12.00 for the exact same scissors you’re making.
In your first year, selling 10,000 units at $12 per unit generates $120,000 in revenue. After all costs (manufacturing, certifications, logistics, partner fees), you’ll net around $35,000 profit. That’s a 117% return on your $30,000 investment. Break-even happens around 12 to 14 months.
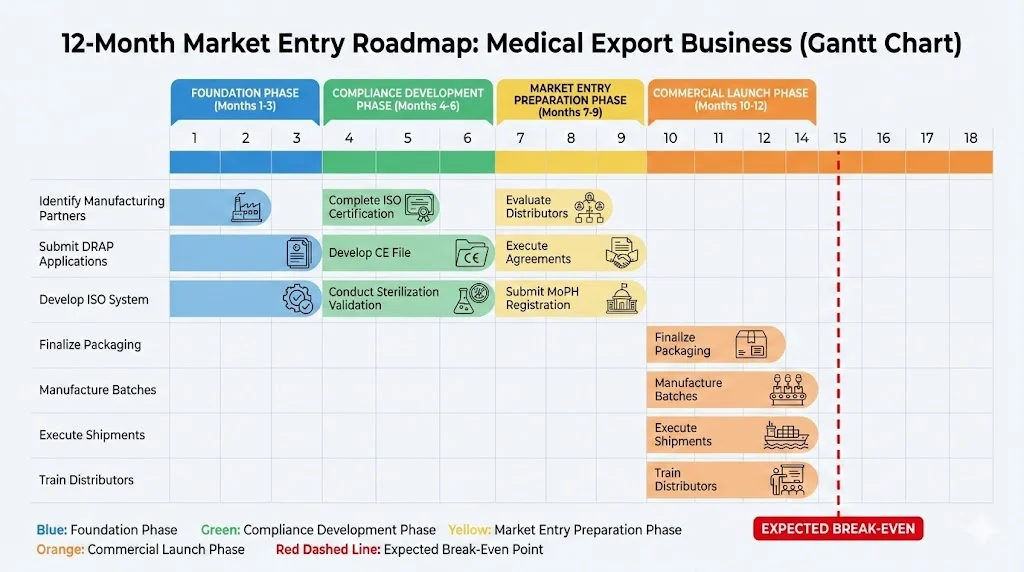
Your Step-by-Step Compliance Roadmap
Here’s where most exporters get stuck. The paperwork seems overwhelming. Let’s make it simple.
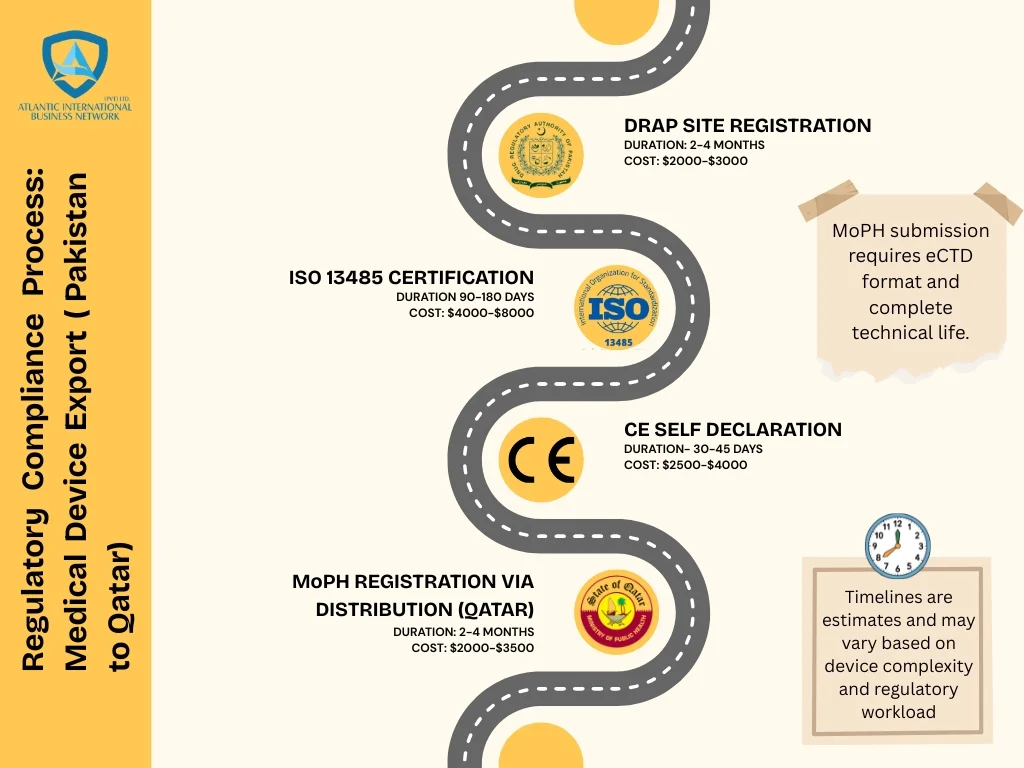
Step 1: Get DRAP approval (Months 1-3)
Start with Pakistan’s Drug Regulatory Authority. Register your manufacturing site or contract with a DRAP-licensed factory in Sialkot. You’ll submit factory layouts, equipment lists, production flowcharts, and cleaning procedures.
DRAP inspectors will visit your facility. They’re checking that you actually follow the procedures you documented. Keep everything clean, organized, and exactly as described in your paperwork. Building strong capacity building programs within your team ensures everyone understands compliance requirements.
Step 2: Earn ISO 13485 certification (Months 4-6)
This is your international quality passport. Hire a certification body like TÜV or SGS. They’ll audit your quality management system in two stages—first reviewing your documentation, then spending days at your facility watching how you actually work.
You’ll need a quality manual, standard operating procedures, batch records, and complaint-handling systems. Sounds like a lot, but it’s mostly about documenting what you already do and following it consistently.
The certification lasts three years but requires annual check-ins. Budget for this ongoing cost.
Step 3: Build your CE technical file (Months 4-6)
Your CE file proves your scissors meet European safety standards. Qatar accepts CE certification, so this document opens their market.
Include material certificates showing your stainless steel meets medical-grade specs, manufacturing flowcharts matching your ISO procedures, mechanical testing results proving sharpness and strength, risk management analysis following ISO 14971, sterilization validation from your provider, and bilingual labels in English and Arabic.
Step 4: Find your Qatar distributor (Months 7-9)
You can’t apply directly to Qatar’s Ministry of Public Health. Only licensed Qatar distributors can submit registration applications.
Look for distributors already selling medical devices to Hamad Medical Corporation. Check their portfolios—do they represent quality brands? Verify their MoPH import licenses are current. Our work with strategic alliance formation has shown that strong distributor relationships make or break market entry success.
Negotiate your agreement carefully. Define territories, set minimum purchase commitments, and establish your pricing structure. Get everything in writing.
Step 5: Secure import permits and market authorization (Months 7-9)
Here’s where Qatar differs from some other markets. Currently, non-implantable Class I devices like surgical scissors don’t require full formal registration—they need import permits and marketing authorization instead.
Your distributor handles this through Qatar’s Ministry of Public Health and Ministry of Economy & Commerce. They’ll submit your application package including your ISO certificate, CE technical file, DRAP Free Sale Certificate, Arabic label proofs, and your signed distributor contract.
The process takes two to four months. Once approved, you’re legal to sell in Qatar. While the process is called “import authorization” rather than “registration” for Class I devices, it still requires the same documentation quality and distributor relationship.
Important note: If you expand into implantable devices later (like surgical screws or plates), those DO require full formal registration with additional scrutiny. Start simple with disposable instruments.
The Hidden Challenges Nobody Warns You About
We’ve worked with 25+ businesses across the Pakistan-GCC corridor over 10+ years. Here’s what trips up new exporters.
Registration delays: MoPH might request additional information or clarifications. This adds weeks or months. Hire an experienced regulatory consultant who knows Qatar’s system. They’ll get it right the first time.
Distributor problems: Sometimes distributors promise the world but don’t deliver. They take your products, then don’t actively sell them. Protect yourself with performance clauses in your agreement and regular sales reviews.
Quality consistency: One bad batch destroys your reputation. Hospitals remember suppliers who shipped defective products for years. Invest in quality control systems from day one. Test every batch before shipping.
Cash flow pressure: You’ll be paying for certifications and inventory months before you see revenue. Most exporters underestimate how long it takes to break even. Keep enough working capital to survive 18 months without profit.
Label compliance: Qatar customs officers are picky about labeling. If your English-Arabic labels aren’t perfect, they’ll seize your entire shipment. Use professional medical translation services and double-check everything.
How AIBN Makes This Journey Easier
Look, you can do all of this yourself. Some exporters do. But here’s what we’ve learned from launching 19+ ventures between Pakistan and the GCC—having someone who’s done it before saves you months and thousands of dollars.
We help Sialkot manufacturers navigate the compliance maze. Our team knows which certification bodies move fast, which consultants actually deliver, and which Qatar distributors have real hospital relationships.
We’ve got direct access to the Qatar Chamber and the Qatar Free Zone. That matters when you need distributor introductions or want to set up meetings with Hamad Medical Corporation’s procurement team.
One of our clients went from concept to first shipment in 11 months. Another saved $8,000 by avoiding consultant mistakes we’ve seen before. Developing strong partnership strategies and understanding policy requirements early prevents costly delays.
We’re not a magic solution. You’ll still do the work. But we’ll make sure you’re working on the right things in the right order.
Your First 90 Days Action Plan
Ready to start? Here’s what to do in the next three months.
Weeks 1-4: Visit three Sialkot manufacturing facilities. Tour their operations, review their quality systems, and get manufacturing quotes. Pick your partner based on quality consistency, not just price.
Start your DRAP application. Gather all required documentation and submit it. Hire a DRAP consultant if this is your first medical device export—they’ll guide you through the process and prevent rejection.
Weeks 5-8: Engage an ISO certification body. Schedule your stage one documentation audit. While waiting, develop your quality management system documentation. Many certification bodies offer template packages that speed this up significantly.
Research Qatar distributors online. Make a shortlist of five potential partners. Look at their websites, check their product portfolios, and verify their MoPH licenses.
Weeks 9-12: Complete your ISO stage one audit and fix any gaps identified. Begin developing your CE technical file with a medical device consultant who understands Class I requirements.
Contact your shortlisted Qatar distributors. Schedule video calls to discuss partnership opportunities. Visit Qatar if possible—face-to-face meetings build trust faster than emails. Understanding market entry requirements and conducting proper competitive analysis gives you negotiating power.
Set up sterilization trials with EO or gamma facilities. You’ll need validation data for your technical file.
The Bottom Line: Is This Worth It?
You’re already making quality surgical instruments—scissors, forceps, scalpels, retractors, whatever your specialty is. Sialkot’s reputation for craftsmanship is solid worldwide. The question isn’t whether you can make good products—you already do.
The question is whether you want to keep selling through middlemen who take 70% of the value, or build your own brand and capture those margins yourself.
Qatar’s healthcare market isn’t slowing down. Hospitals need continuous supplies of disposable instruments across every category. The regulatory framework is clear and achievable. The investment is manageable for serious manufacturers.
We’ve seen this work over and over. Pakistani manufacturers who commit to compliance, invest in quality systems, and build real relationships in Qatar succeed. Those who try to cut corners or rush the process fail.
The opportunity is real. The path is clear. Whether you start with scissors, forceps, or any other instrument—the framework is the same. The only question left is whether you’ll take the first step.
Ready to Export Surgical Scissors to Qatar?
Stop watching European distributors profit from your hard work. Take control of your export journey.
We’ve helped 19+ businesses successfully navigate the Pakistan-GCC corridor. Our team knows exactly what works and what doesn’t when you want to export surgical scissors to Qatar.
Schedule a consultation with AIBN today. We’ll review your current capabilities, map out your specific timeline, and show you exactly what it takes to land your first Qatar contract. Visit our connect page to get started, or explore our complete range of market entry solutions.
Your scissors deserve to carry your brand. Let’s make that happen.